Water Vapor Formula
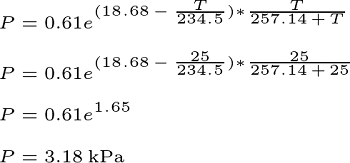
Water vapor, the gaseous state of water, plays a crucial role in Earth’s atmosphere, climate systems, and various industrial processes. Understanding its behavior and properties requires a grasp of its chemical formula and the underlying principles governing its interactions. This article delves into the water vapor formula, its significance, and its applications across different domains.
The Chemical Formula of Water Vapor

The chemical formula for water vapor is identical to that of liquid water: H₂O. This formula represents one molecule of water, consisting of two hydrogen atoms (H) covalently bonded to one oxygen atom (O). In the gaseous phase, water molecules exhibit greater kinetic energy and are more dispersed compared to their liquid or solid counterparts.
Expert Insight: While the formula remains H₂O, the physical state of water (solid, liquid, or gas) is determined by temperature and pressure conditions. Water vapor forms when liquid water molecules gain sufficient energy to break free from intermolecular forces and transition into the gas phase.
Phase Transitions and Water Vapor

Water vapor is intimately linked to phase transitions, particularly:
- Evaporation: The process by which liquid water transforms into water vapor at temperatures below its boiling point.
- Boiling: The rapid conversion of liquid water into vapor at its boiling point (100°C or 212°F at standard atmospheric pressure).
- Condensation: The reverse process, where water vapor cools and returns to the liquid state, forming droplets or dew.
Steps in the Evaporation Process:
- Heat is absorbed by liquid water molecules, increasing their kinetic energy.
- Molecules at the surface overcome intermolecular forces and escape into the air as vapor.
- The rate of evaporation depends on temperature, humidity, and surface area.
Role of Water Vapor in the Atmosphere
Water vapor is the most abundant greenhouse gas in Earth’s atmosphere, accounting for approximately 60-70% of the natural greenhouse effect. It absorbs and re-emits infrared radiation, trapping heat and regulating the planet’s temperature. Key aspects include:
- Humidity: A measure of water vapor content in the air, expressed as relative humidity (percentage of saturation) or absolute humidity (mass of water vapor per volume of air).
- Cloud Formation: Water vapor condenses into liquid droplets or ice crystals, forming clouds.
- Precipitation: When condensed water droplets grow large enough, they fall as rain, snow, or other forms of precipitation.
Key Takeaway: Water vapor is a critical component of the hydrological cycle, driving weather patterns and climate dynamics.
Industrial and Practical Applications
Water vapor’s unique properties make it indispensable in various industries:
- Power Generation: Steam (water vapor under pressure) drives turbines in thermal power plants, generating electricity.
- Chemical Processes: Water vapor is used as a reactant or solvent in numerous chemical reactions, such as hydrogen production via steam reforming of methane.
- Humidification: Controlled addition of water vapor to air in HVAC systems improves indoor air quality and comfort.
| Industry | Application |
|---|---|
| Energy | Steam turbines for electricity generation |
| Chemical | Steam reforming for hydrogen production |
| Textile | Humidification to prevent static and fiber damage |

Measuring Water Vapor

Accurate measurement of water vapor is essential for meteorological, industrial, and scientific applications. Common methods include:
- Psychrometers: Measure relative humidity by comparing dry and wet bulb temperatures.
- Hygrometers: Directly measure humidity levels using sensors like capacitive or resistive elements.
- Spectroscopy: Analyzes water vapor concentration in atmospheric samples using infrared or microwave sensors.
Advantages and Limitations of Measurement Techniques:
- Pros: Psychrometers are simple and cost-effective; spectroscopy provides high precision.
- Cons: Psychrometers require manual operation; spectroscopy is expensive and complex.
Environmental and Climate Implications
Water vapor’s role in climate change is complex and multifaceted. While it is a natural greenhouse gas, its concentration is influenced by human activities, such as:
- Deforestation: Reduces transpiration, lowering local humidity.
- Industrial Processes: Release water vapor as a byproduct, potentially altering regional climates.
- Feedback Loops: Warmer temperatures increase evaporation rates, amplifying the greenhouse effect.
"Water vapor is both a consequence and a driver of climate change, making it a critical variable in climate models." – Intergovernmental Panel on Climate Change (IPCC)
What is the difference between water vapor and steam?
+Water vapor refers to gaseous H₂O at any temperature, while steam specifically denotes water vapor at or above its boiling point (100°C or 212°F), often under pressure.
How does water vapor contribute to global warming?
+Water vapor amplifies the greenhouse effect by absorbing and re-emitting infrared radiation. Its concentration increases with rising temperatures, creating a positive feedback loop.
Can water vapor be used as a renewable energy source?
+While water vapor itself is not a direct energy source, steam derived from renewable heat sources (e.g., solar thermal) can be used to generate electricity.
What is the maximum amount of water vapor air can hold?
+The maximum water vapor capacity of air depends on temperature. Warmer air can hold more moisture; for example, at 30°C, air can hold approximately 42 grams of water vapor per cubic meter.
Conclusion
The water vapor formula, H₂O, encapsulates the essence of this vital compound. From its role in Earth’s climate to its applications in industry, water vapor’s significance cannot be overstated. Understanding its properties, behavior, and measurement techniques is essential for addressing environmental challenges and harnessing its potential in technological advancements. As we continue to explore and study water vapor, its importance in both natural and engineered systems will only grow.